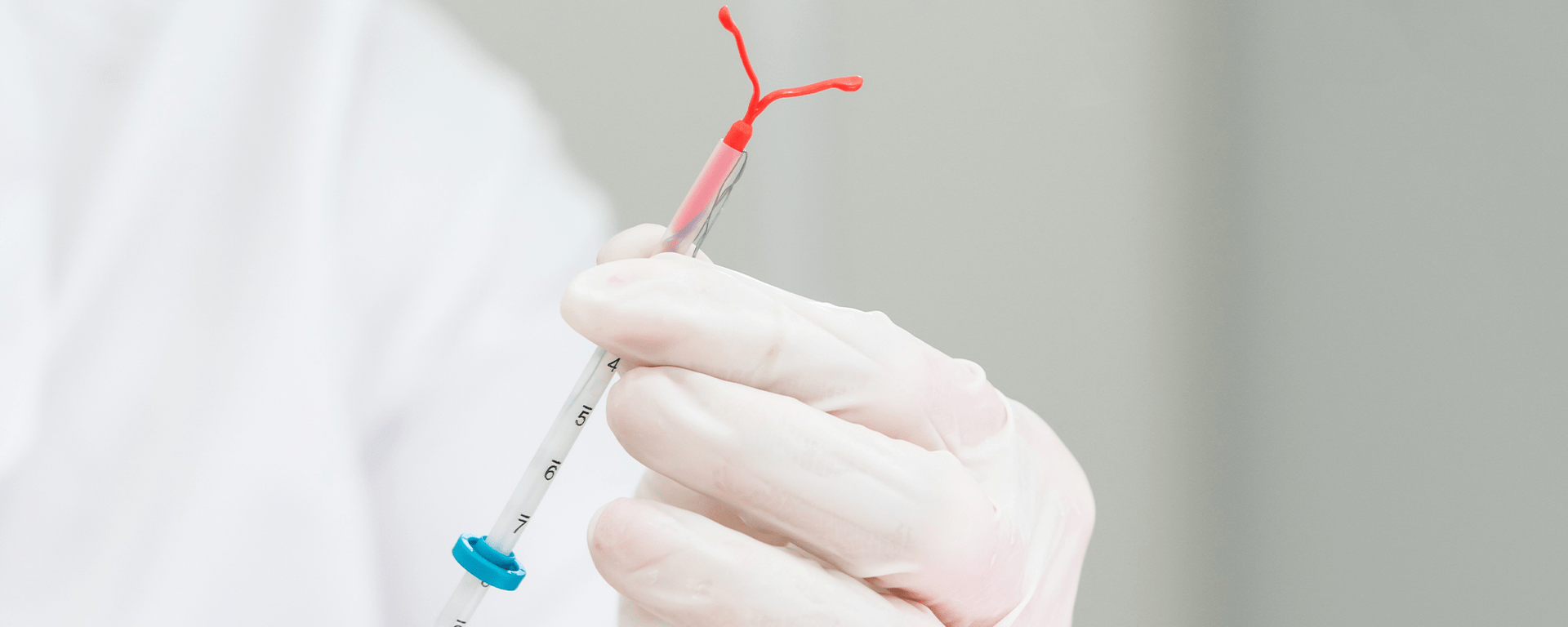
PS: Pessoal, este é um artigo que escrevi para o meu Mestrado em Biotecnologia e Empreendedorismo na Universidade de NY. Para aqueles que tiverem curiosidade de saber a composição e como os sistemas intra-uterinos liberam hormônios.
Fernanda Pacheco, M.D.1
1New York university Tandon School of Engineering – Master student of Biotechnology and Entrepreneurship
Abstract
Unintended pregnancies are a relevant issue around the world. In the U.S. around 50% of pregnancies were not planned. Among the contraceptive methods, the long acting reversible contraceptives (LARCS: subdermal implant and intra-uterine devices (IUDs)) have been gaining emphasis due to their convenience, safety, effectiveness and cost-effectiveness after 20101.
MirenaÒ (Bayer Healthcare Pharmaceuticals, Whippany, NJ, USA)was the first hormone-releasing intra uterine device (IUD) approved by the U.S. food and drug administration (FDA). Currently, it is the most prescribed IUD in America. Besides the indication of birth control, the method can be indicated to treat heavy menstrual bleeding (HMB) as well. The device can be maintained in the uterine cavity for up to 7 years to avoid pregnancy and for up to 5 year to treat heavy periods. MirenaÒ is composedby silicone, polyethylene, silica, barium sulfate and iron oxide and a progesterone named levonorgestrel is released. The goal of this study is to discuss the physicochemical properties and the biocompatibility of the biomaterials present in the IUD. For this review, first, the author verified the package insert of the product to find out which materials compose the contraceptive device according to its manufacturer company. Then, I reviewed the literature, the material of the course Biomaterials and Devices for Human Body Repair in the Master of Biotechnology and Entrepreneurship at New York University Tandon School of Engineering, and the American Society for Testing and Materials (ASTM) to list the characteristics of each material.
Introduction
Unintended pregnancy is a relevant concern worldwide with multiple negative aspects for the parents, children, and the society as a whole. It affects not only undeveloped countries as well as in developed ones. For instance, of the 6 million pregnancies that occur every year in women from 15 to 44 years of age in the United States, about half of them are unplanned. Although numbers have decreased among rich and highly-educated women between 1994 and 2001, the same is not true for the poor and not-educated population2. Moreover, the great majority of unplanned pregnancies, happen among teenagers, who tend to have repeat undesired pregnancies and tend to be daughters of mothers who also gave birth to them during adolescence. Another concerning fact is that around half of unintended pregnancies occurred during the use of contraceptives (contraceptive pills, injections, vaginal ring, patches), not because fail of the methods, but because of misusage, since they depends on a correct administration2.
In 2010, the results of a conduct-change paper, named “CHOICE Project” were published1. The aim of this study was to promote access for the insertion of long acting reversible contraceptives (LARCS), eliminating financial and knowledge barriers among 10,000 women who desired contraception for at least 1 year in Saint-Louis. At that time, despite convenience of use, proof of safety, and high effectiveness, LARCS (subdermal implant, cupper intra-uterine device (IUD), hormone-releasing intra-uterine systems) were used for less than 3 % of American women. The project proved that once financial barriers were removed, MirenaÒ (Bayer Healthcare Pharmaceuticals, Whippany, NJ, USA), the first hormone-releasing IUD approved by the U.S. food and drug administration (FDA) was the first option for contraception among women from 14-44 years in the study in question. Additionally, the study proved that IUDs were safe even for adolescents, knocking down the concept that they could not be used in nulliparous patients.
MirenaÒ was introduced in the American market in 2000, 10 years later its launch in Europe, because IUDs were very unpopular in the U.S. in the 90’s due to Dalkon ShieldÒ (A.H. Robins Company, U.S.) lawsuits. That IUD was commercialized in the U.S. and Puerto Rico between the 70’s and 80’s and caused several cases of pelvic infection and some deaths in its users, probably because the biomaterial used in the filaments attached to the base of the IUD used to pull it out. Therefore, a long road was crossed for today MirenaÒ being the most prescribed IUD in the U.S.
In this study, I will discuss the physicochemical properties of the active (LNG) and inactive ingredients present in MirenaÒ (silicone, polyethylene, silica, barium sulfate and iron oxide according to the manufacturer company). For this review, the author reviewed manufacturer information, including the package insert of the IUD and the literature (Google Scholar, Pubmed, National Library of Medicine).
Results
MirenaÒ consists in a T-shape polyethylene frame of 1.26 inches or 32 mm both vertically as horizontally, containing barium sulfate for radio-opacity purpose. Around the stem, there is a white cylindrical hollow reservoir, made by a mixture of 52 mg of LNG and polydimethylsiloxane (PDMS) polymer covered by a semi-opaque silicon membrane of PDMS that controls drug release3. Besides the arms, the steam, and the hormone reservoir, the IUD possesses two brown monofilament threads of polyethylene, with iron oxide used as colorant3, attached to a loop in its lower extremity (Figure 1), which serves both for patient to control the device position through intravaginal touch and for health professionals to pull the device out. MirenaÒ is classified as a non-biodegradable passive implant, in other words, the IUD contain non-biodegradable polymers that serve as a diffusional barrier to yield drug flux. Reservoir-type systems like MirenaÒ separate a drug compartment from a polymer membrane. These types of systems have the benefit of maintaining a constant drug release rate that is not affected by concentration gradient, but most likely is related to the membrane thickness and the permeability of the drug through the polymer (zero-order release kinetics). The system is light, flexible, ductile, though, and biocompatible, with rare cases of reported allergies to one of its components: silicone, polyethylene, silica, barium sulfate and iron oxide. Although non-biodegradable polymers are considered biocompatible, they can cause infection, tissue damage or cosmetic disfigurement. Wherefore, once all the drug has been released, the system must be removed to prevent any side effects4.
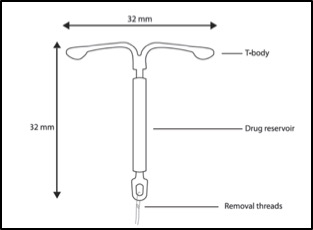
Figure 1. MirenaÒ Diagram. Extracted from manufacturer company website https://www.mirena-us.com/about-mirena/mirena-effectiveness
Levonorgestrel (LNG)
LNG is poorly soluble in water with a LogP = 3.85. Its chemical formula is C21H28O2 (Figure 2) and molecular weight is 312.4.
Additionally,LNG is commonly used in birth control methods as combined hormonal oral contraceptives, emergency contraceptives, subdermal implants and IUDs (MirenaÒ,KyleenaÒ, JaydessÒ). MirenaÒ is loaded with 52 mg of levonorgestrel, a progesterone hormone that is released in a dose of approximately 20 mcg in a daily basis in the first 3 months, reduced to about 18 mcg/day after the first year, to about 10 mcg/day after the fifth year and to 8 mcg/day after the seventh year of use. The amount of LNG in the studied system is substantially high and potentially toxic in case of dose dumping or unanticipated release rate in vivo. Therefore, authors from a study of 20185 state that the device represents a challenging manufacturer and that until the publication date there were no reports on manufacturing, product design and quality other than the MirenaÒ patent, basically due to the long-time of investment, research, and development of the product (25 years), the long-acting, and the high cost of MirenaÒ cycle. The first patent of MirenaÒ came off patent in 2015, but as of 2018 no other pharmaceutical company had launched a similar product; hence, authors claimed for the need of a reproducible and robust manufacturing process for the understanding of this long-acting IUD in vitro and in vivo performance. Then, authors created a twin-syringe device similar to MirenaÒ in order to understand material-mold, curing (temperature and time) and drug loading, three aspects very important for the LNG-releasing-IUD quality.
PDMS
PDMS [(CH3)2SiO]n is the simplest silicon and consists in a repetition of -Si(CH3)2-O- .It is a polymer obtained by hydrolysis with chemical structure based on silicon rather carbon alternated with oxygen and organic chains linked to the silicon base. Its hydrocarbon component repeals water. Silica is a complex material present in food and pharmaceuticals.
It is non-toxic, stable, which means, without chemical reaction, an electricity insulator, and flame resistant. This polymer has been widely used in medical devices, particularly in contraceptives (subdermal implants, transdermal devices, vaginal rings) for controlling drug release, due to its thermal-stability, chemical inertness, elastomeric properties, long-term biocompatibility, resilience and robustness. The silicones commonly used in Medicine are vulcanized in room temperature and are prepared using two-component poly(dimetylsiloxanes) in the presence of a catalyst (platinum-based compound). The final material is formed via an addition hydrosilation reaction. The diffusivity of the drug through the PDMS is crucial to control the dose. PDMS is controlled as a crosslinked network, as a result, the crosslinked density will influence water uptake of the material and the diffusivity of the drug. Accordingly, these parameters should be optimized in order to obtain the required drug release profile.
Polyethylene
The polyethylene chemical structure is based on carbon. It is a low-cost polymer, an excellent electricity insulator, resistant to chemical exposure, flexible and though.
Barium Sulfate
Barium sulfate (or sulphate) is an inorganic component, BaSO4, insoluble in water, and opaque. It increases density and resistance to acid and alkali in polymers and it is used for XR-opacity due to its radio-opacity. A very important property since the device is not clearly visualized by ultrasound and perforation of uterus and migration to the abdomen cavity is not a rare complication after insertion.
Iron Oxide
As aforementioned, it is used in the two filaments of MirenaÒ to give them the brown color.
Conclusion:
Recent studies have showed that MirenaÒ could have the length of use prolonged from 5 to 7 years, because the system continued releasing LNG after the fifth year and that the biomaterials compounded in it were resistant to the extended period.
Discussion
In the near future hormone-releasing IUDs will be manufactured in a more personalized pattern with 3D printing, allowing the confection of an IUD specific for each woman, according to their size, required dose of hormone released daily in a steady rate, and requested period of contraception. If it is possible that materials be all degradable it is even better because it skips the removal step, which in general is very easy, but sometimes can be tough, requiring even the need of being removed at the Hospital under sedation in situations where the threads disappeared into the cervix or the material got incrusted in the uterus.
References
1. Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. Aug 2010;203(2):115.e1-7. doi:10.1016/j.ajog.2010.04.017
2. Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. Jun 2006;38(2):90-6. doi:10.1363/psrh.38.090.06
3. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/021225Orig1s033.pdf.
4. Sarah A. Stewart JD-R, Ryan F. Donnelly and Eneko Larrañeta. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. 2018;10(1379):1-24.
5. Bao Q, Gu B, Price CF, et al. Manufacturing and characterization of long-acting levonorgestrel intrauterine systems. Int J Pharm. Oct 25 2018;550(1-2):447-454. doi:10.1016/j.ijpharm.2018.09.004
6. KR M. The chemistry of silica and its potential health benefits. . Mar-Apr;11, 2007; (2)::94-7. doi:PMID: 17435951.